Saturated And Unsaturated Solutions Worksheet Answers
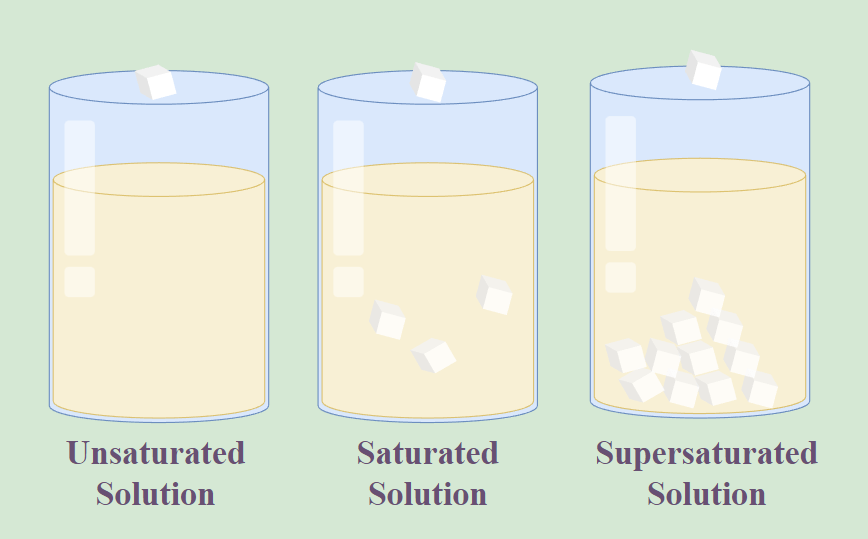
Saturated And Unsaturated Solutions Worksheet Answers - Web different solutes, such as salt, sugar, or minerals, dissolve to very different extents in water (and other solvents). Worksheets are topic 4 solutions, chem 12 chapters 1. Dissolve lots of solute in it. • solute is the same. A solution k is saturated at 33 g per 100 g. Web model 1 — saturated and unsaturated solutions unsaturated solutions all beakers contain 10.0 g of water. How does a solution become supersaturated? • all solutions are stirred for 2 hours. How will you test whether a given solution is saturated or not? Dissolve a little solute in it.
Web check your knowledge of the features of unsaturated solutions with an interactive quiz and printable worksheet. Dissolve a little solute in it. [figure 2] when 30.0 g of nacl is added to 100. (b) how would you prepare a saturated. You need to stir it more. In this activity you will learn how to quantify the amount of solute. A solution in which more of the solute can be dissolved at any given temperature is called an unsaturated solution.
Web check your knowledge of the features of unsaturated solutions with an interactive quiz and printable worksheet. Web an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Two materials have combined to create a. How does a solution become supersaturated? Label one glass ‘salt’ and the other glass.
• all beakers are kept at 20 °c. • solute is the same. Web check your knowledge of the features of unsaturated solutions with an interactive quiz and printable worksheet. How does this resource excite and engage children's learning? How does a solution become supersaturated? All solutions are stirred for 2.
How does a solution become supersaturated? Some of the worksheets for this concept are topic 4 solutions, chem 12 chapters 10 11 12 13. • all solutions are stirred for 2 hours. Worksheets are topic 4 solutions, chem 12 chapters 1. When 40.0g 40.0 g is added, 36.0g 36.0 g.
How will you test whether a given solution is saturated or not? Label one glass ‘salt’ and the other glass. These practice questions will help. Fill each glass with equal amounts of water (about 200ml should be enough).
Web Bottom Filter The Solution And Mass The Remaining Solid, If The Mass Is The Same Then The Solution Was Saturated, If The Mass Is Lower Than It Is An Unsaturated Solution.
• all beakers are kept at 20 °c. [figure 2] when 30.0 g of nacl is added to 100. Dissolve lots of solute in it. Web model 1 — saturated and unsaturated solutions unsaturated solutions all beakers contain 10.0 g of water.
Some Of The Worksheets For This Concept Are Topic 4 Solutions, Chem 12 Chapters 10 11 12 13.
When a solution is saturated: When 40.0g 40.0 g is added, 36.0g 36.0 g. Web different solutes, such as salt, sugar, or minerals, dissolve to very different extents in water (and other solvents). • solute is the same.
Web An Unsaturated Solution Is A Solution That Contains Less Than The Maximum Amount Of Solute That Is Capable Of Being Dissolved.
Dissolve a little solute in it. All solutions are stirred for 2. When 30.0g 30.0 g of nacl nacl is added to 100ml 100 ml, it all dissolves, forming an unsaturated solution. • all beakers are kept at 20 °c.
• All Solutions Are Stirred For 2 Hours.
You need to stir it more. A solution k is saturated at 33 g per 100 g. Web (a) differentiate between a saturated and an unsaturated solution. How does a solution become supersaturated?